Microbiome and Body Mass Index: Who Benefits from Whom?
What are the general differences in the gut microbiota of people with different body mass indices?
For the guts of leaner people, a richer microbial diversity is characteristic. It mostly reflects the diversity of diet: a wider range of foods, and accordingly, the groups of microorganisms that consume them. The degree of microbial community diversity reflects its resilience to various catastrophes, such as food poisoning and infections.
There is a lot of data regarding the relationship between body mass and the ratio of the number of bacteroides and firmicutes. Currently, the overwhelming majority of studies say that the more Firmicutes and the fewer Bacteroidetes, the more likely problems with overweight. However, [there are also other studies (not many in number)] that claim the opposite, or even indicate the absence of such a connection.
Many researchers also note a greater number of bifidobacteria and Lactobacillus plantarum in leaner people, while the rarely mentioned Christensenellaceae were more frequent residents in the guts of heavier participants.
How might the gut microbiota influence the degree of weight gain?
Interestingly, people with different body mass indices show differences in how efficiently they metabolize food. And this directly depends on our microbiota. In studies it has been shown that when consuming excessive amounts of food, lean participants in experiments had lower energy extraction efficiency, unlike heavier individuals.
About the dark side of short-chain fatty acids
It is known that about 10% of our energy reserve is obtained from the digestion of dietary fiber by our bacteria. As a result, short-chain fatty acids (short‐chain fatty acids, SCFAs) are formed, which are important signaling molecules and a source of energy for both the intestinal epithelium and the liver and peripheral tissues. In some studies it has been shown that in patients with obesity elevated SCFA values were observed, from which it is inferred that this energy bonus could contribute to weight gain. That is, these wonderful and beneficial short-chain fatty acids can also be used as substrates for lipid synthesis. Thus, the share of fermented fiber and the possible energy gain that can be "drawn" from the consumed food largely depends on the “abilities” of our gut community and a bit on the sense of humor of our metabolism. In simple terms, after eating a plate of porridge, we all obtain a different amount of calories from it.
About the bugs of the “smart litter”
The permeability of the intestinal barrier and its selectivity are not trivial. We need to extract nutrients, water, vitamins, trace elements from the gut contents, but not to accumulate something extra and excessive. Microbial metabolites, and even the microbes themselves, can cross the gut barrier and enter the bloodstream. When this “border control” fails and lets through an excess of microbial lipopolysaccharides (components of the cell walls of Gram-negative bacteria, such as E. coli), our immune system responds by developing a state of chronic inflammation, which is an indispensable component of the development of obesity and type 2 diabetes. A trigger for this can be stress. It causes an increase in the same inflammatory markers in the blood and disrupts that “smart gate” by releasing lipopolysaccharide molecules from the intestine, further promoting systemic inflammation and the development of inflammatory processes in the gut.
About mind games
Microbial metabolites interact with our nervous system and influence hormone production. Among them are hormones that identify our feeling of fullness or hunger. This is already a bit like the plot of a scary film: in some studies published in Nature in 2016 it was shown that when feeding test rats a fatty diet, their microbiota produced a lot of acetate. It interacted with the parasympathetic nervous system and increased the level of ghrelin (the very hormone that makes us feel hungry). In poor animals, a breakdown occurred; they ate even more, became fatter, developed insulin resistance, and metabolic syndrome.
It seems that the answer to many philosophical questions is right in these results. If you eat a lot of fat and constantly crave more, perhaps you should reflect...
About the wheel of Fortune and bile
Part of our microbial brotherhood deftly ferments bile acids. The more bile is fermented, the less it returns to the bloodstream and can be used again. And more energy currency that could settle on our hips goes into synthesizing new bile. If there are not enough “brothers” who can ferment bile, shedding excess calories in this way is not possible. In addition to such a fairly direct influence on draining excess calories, there are other effects, for example, through FXR signaling (microbial bile acid metabolites interact with FXR receptors and promote the release of FGF-19 (fibroblast growth factor 19), which regulates not only bile acid synthesis but also lipid and glucose metabolism).
And now to a down-to-earth and practical question that many are curious about.
Will there be a super-pill?
Probably. And expensive. Honestly — cheaper to simply eat less and more quality food if the issue is mainly excessive food intake and insufficient activity. No yogurt or pill will turn anyone into a lean deer if they lie on the couch and absorb food like a deer.
But if pills can help motivate, why not? And many products work exactly that way! However, if we set aside some skepticism, specialized preparations based on new microbial players in the probiotic industry could become a good adjunct to cholesterol-lowering and anti-diabetic therapy, and somewhat ease the path to desired sizes.
Currently, studies show that consumption of Bifidobacteria reduced weight gain, cholesterol levels, the amount of proteobacteria (which are a source of lipopolysaccharide), and, accordingly, led to a decrease in markers of general inflammation in mice fed a high-fat diet.
There are also many studies demonstrating weight loss with probiotics containing lactobacilli.
It is important to note that if we turn to probiotics for a specific purpose (in this case, to reduce body weight), we should pay attention to the strain numbers contained in these particular probiotic preparations and check in scientific sources whether these strains are related to weight regulation. We wrote about choosing probiotics in more detail here.
Many of our gut residents exert anti-inflammatory effects, which later has a "weight-loss" effect and will undoubtedly be used by the pharmaceutical industry. For example, relatives of Clostridia, Faecalibacterium prausnitzii, produce butyrate and thereby block the activation of NF-kB and the subsequent release of pro-inflammatory cytokines in intestinal cells.
Another well-known, promising, and widely discussed type of microorganism to which great importance is attached in the near future in the fight against obesity and insulin resistance is the already-mentioned species Akkermansia muciniphila. It helps maintain the barrier function of the gut and already has a number of successful proofs of its effectiveness when used for patients with elevated body mass index.
One of the latest publications on this topic shows that daily consumption of Akkermansia muciniphila at a quantity of 1010^ CFU for three months reduced blood cholesterol by about 8%, body weight by an average of 2 kg, thigh volume by about 2 cm, and also reduced inflammatory marker levels.
However, on the horizon of applying these superhero bacteria, there are small "clouds": some data show an indirect involvement of A. muciniphila in the onset of relapsing multiple sclerosis.
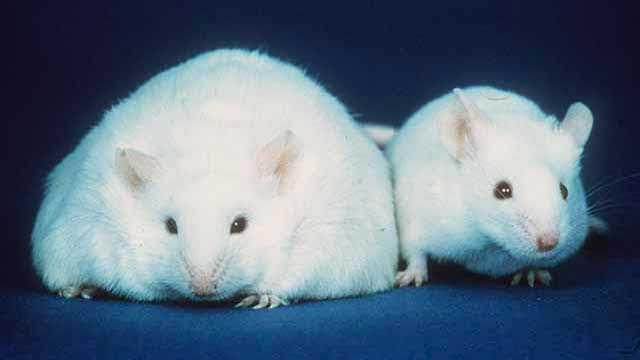
So what about the mice?
Back in 2013, Science published the same article in which samples of the gut microbiome from lean and obese donors were transplanted into germ-free mice. The mice started to gain weight or stayed lean depending on whose microbiome they received. There was another modification of that experiment mentioned less often. Five days after transplantation, mice with microbiomes from lean and obese donors were placed in the same cage, and the mice that were supposed to gain weight did not gain weight! The researchers controlled the quantitative and qualitative changes of the microbiomes in the animals in the cages and noted that there was a transfer of Bacteroides from Lean mice to Fat mice, which resulted in them not becoming fat. Thus, the lean microbiome prevailed. It is also noted in the discussion that the mice are somewhat prone to coprophagy, so it is quite possible that they contributed to this by eating feces of their cage mates. However, the researchers did not state that this is exactly what happened. Considering that the experiment was performed on animals and likely involved consumption of feces, it is unlikely to confidently extrapolate these results to humans. However, there is no reason to worry that you can now acquire an obese microbiome and get fat, it seems unlikely at the moment. More likely, your bifidobacteria will be stronger! However, this should not be a reason to eat poop. In all meanings of this word!
To be continued :)